Tips for Selecting Conjugation Methods
Antibodies are used to detect and quantify antigens using the appropriate detection techniques, such as flow cytometry, ELISA, Western blot, immunofluorescence, and immunohistochemistry. Often for signal amplification and detection purposes, purified antibodies are conjugated to enzymes, fluorophores, or haptens, such as horseradish peroxidase (HRP), alkaline phosphatase (AP), rhodamine, fluorescein isothiocyanate (FITC), or biotin. Labeling strategies result in the covalent attachment of molecular labels to the target protein in order to facilitate the detection of a labeled protein and its binding partners. Labeling assays are classified as ‘direct’ if the label is conjugated to the primary antibody or ‘indirect’ if the label is attached to another molecule, called a ‘secondary reagent’. While multiple types of labels are available, their diverse uses are preferable for specific applications. Therefore, the type of label and the labeling strategy used must be considered carefully and tailored for each application:
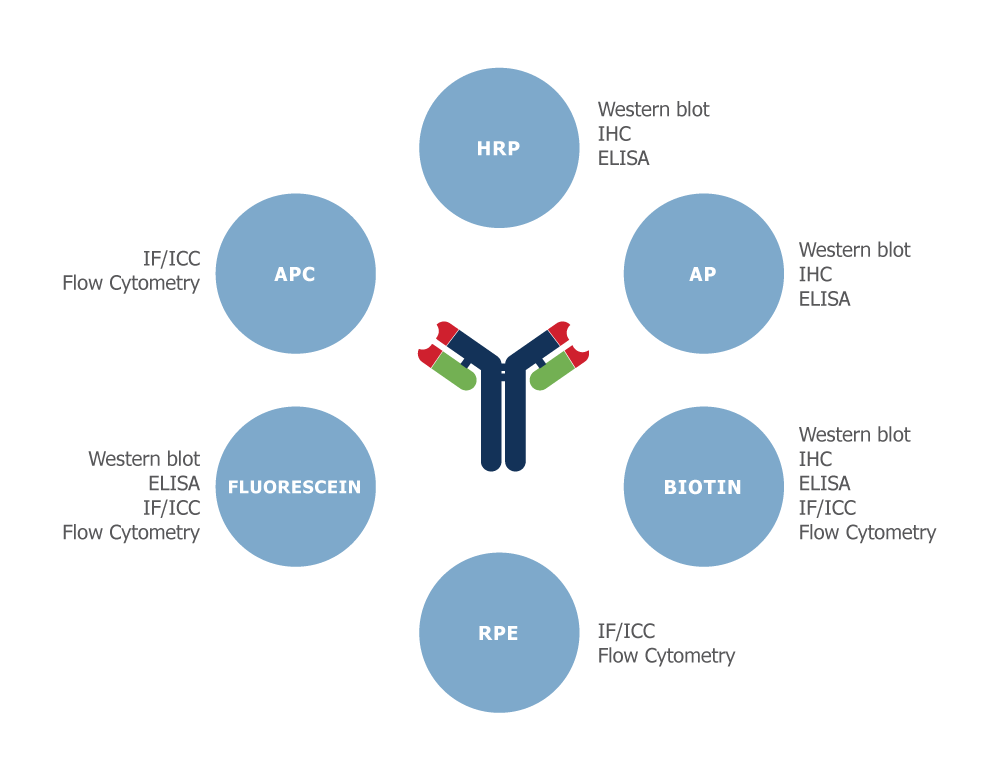
-
Direct or Indirect Detection
The choice of whether to use direct or indirect detection is often dictated by the level of antigen expression. The detection of a highly expressed antigen might be possible using a primary antibody directly conjugated to a label. The direct labeling approach is simple and avoids the problems of non-specific binding with labeled secondary antibodies. Additional advantages of using a conjugated primary antibody is the ability to multiplex with antibodies from the same species, reduce the number of incubation and wash steps, and produce better data quality.
-
Use directly conjugated antibodies only for the detection of very abundant target proteins as direct detection lacks the signal amplification step, which could result in weak or no signal if the target protein is present at low levels.
Indirect detection methods generally have a higher level of sensitivity and generate a more intense signal. The signal is amplified because several secondary antibodies carrying multiple labels bind to the primary antibody. -
Although labeled secondary antibodies are readily available, using them may compromise the required specificity and require extra blocking and wash steps and additional controls.
-
-
Fluorescent Protein Conjugation
Fluorescence detection is based on the use of fluorophores that have unique and characteristic spectra for absorption and emission—they emit a photon at one wavelength when excited by light of another shorter wavelength. The fluorochrome can be conjugated directly to the primary or secondary antibody. Fluorescent-dye conjugated antibodies provide a much-needed tool for identifying proteins in many applications including fluorescent cell imaging, western blotting, immunohistochemistry, and more.
-
The advantages of using a fluorescent-labeled antibody include brighter signal, multiplexing capabilities, and ease of use (many are available pre-conjugated to many different colors of dyes).
Fluorescein derivatives and their conjugates are the most common fluorescent reagents for biological research as they encompass several performance characteristics such as high absorptivity, excellent fluorescent quantum yield, and good water solubility. When choosing fluorophores, the excitation and emission spectra of each fluorophore should be considered for each experiment. It is important to avoid overlapping emission spectrums if co-localization of two different proteins is desired. -
Select fluorophores with high extinction coefficient—the higher the extinction coefficient, the brighter the fluorophore. Also select fluorophores with high quantum yields, which is a read-out of the efficiency of the fluorescence process.
Photobleaching is a photochemical process that reduces the intensity of the fluorescence signal—FITC and R-phycoerythrin are known to have a relatively high rate of photobleaching. Many conventional fluorophores, such as FITC, are not suggested for staining protocols using acidic buffers as the fluorescence intensity signal is greatly sensitive to an acidic environment. Use new generation dyes that stay fluorescent over a broad pH range. -
If performing multi-color immunofluorescence experiments, use fluorophores with narrow emission spectra in order to avoid spectral overlap or bleed-through (detection of one fluorophore in another fluorophore’s filter set). Bleed-through makes it difficult to detect distinct fluorescence signals and complicates the assessment of co-localization experiments. Ideally, there should be no spectral overlap between the fluorophores.
In order to verify that the observed fluorescence is a result of staining rather than an unspecific artifact, it's important to use the appropriate controls with each experiment. As cells can display natural fluorescence, it is essential to check immunofluorescent samples microscopically before every staining experiment. Additionally, a label/fluorophore control should be included by performing the complete staining protocol without the addition of fluorophore-conjugated antibodies. Use positive and negative controls (for example cell lines in which your protein of interest is either over-expressed or absent such as a knock-out cell line) with each experiment.
-
If using secondary antibodies rather than directly fluorophore-conjugated primary antibodies, a secondary antibody-only control should be performed following the same protocol without the addition of a primary antibody. This will verify that the secondary antibody does not non-specifically bind to certain cellular compartments.
-
For multi-color immunofluorescence experiments, use cross-adsorbed/pre-adsorbed secondary antibodies as these will minimize the risk of the secondary antibody reacting with endogenous immunoglobulins or an undesired primary antibody.
-
-
Enzyme Protein Conjugation
To facilitate chromogenic detection, the primary antibody or secondary antibody is conjugated to an enzyme. The enzyme reacts with a soluble organic substrate to generate an insoluble, colored product that is localized to the sites of antigen expression. Chromogenic or precipitating substrates offer the simplest and most cost-effective method of detection. Various reporter enzymes, such as HRP, AP, and many others, can be attached to antibodies and proteins through the use of different coupling chemistries to ensure the maximum retention of activity of both enzyme and protein. HRP can be visualized by chromogenic reactions. For example, diaminobenzidine (DAB), tetramethylbenzidine (TMB), and AP signal is often measured through its colorimetric substrate p-Nitrophenyl Phosphate, Disodium Salt (PNPP). These enzyme-antibody conjugates can be used in applications such as ELISAs, blotting techniques, in situ hybridization, cytochemistry, and histochemistry detection system.
Peroxidase is an economical and more stable enzyme than alkaline phosphatase. It has also become very popular for use in chemiluminescent detection systems. Alkaline phosphatase, on the other hand, is considered more sensitive than peroxidase particularly when colorimetric detection is used. Chromogenic substrates exhibit low sensitivity and thus it is difficult to optimize them for detecting proteins of low abundance. Although the reaction can be allowed to develop for several hours or even overnight, this leads to increased background signal.
- Use chromogenic substrates for applications where protein abundance is high. Chromogenic detection is considered to be more sensitive than immunofluorescence but is less convenient because it includes more incubation and blocking steps.
Like immunofluorescence, chromogenic detection allows for the visualization of multiple antigens, but only if the antigens are localized at different sites in the cell or tissue because overlapping colors may obscure results. DAB chromogenic staining should be used if slides need to be stored for longer periods as the colored precipitate formed during the reaction between HRP and DAB is not sensitive to light.
-
Biotin/Streptavidin Conjugation
Biotin/Streptavidin is commonly used when the target of interest is expressed at low levels and cannot be detected using labeled antibodies alone. Biotin is used in two-step detection systems in concert with conjugated streptavidin or avidin. Many biotin molecules can be conjugated to an antibody with the additional advantage of binding to streptavidin and avidin with extremely high affinity, fast-on-rate, and high specificity. Through this amplification step and having the streptavidin bound to labels such as HRP or fluorescent probes, proteins expressed at low levels are more likely to be detected.
Streptavidin-based amplification techniques are commonly used in flow cytometry, WB, IF, and microplate-based detection for increased signal and greater sensitivity. Fluorescent conjugates of streptavidin are used to detect biotinylated macromolecules such as primary and secondary antibodies, ligands and toxins, or bead-based detection. HRP and AP enzyme conjugates of streptavidin are commonly used in WB, ELISA, and in situ hybridization imaging techniques. Streptavidin-conjugated magnetic beads are used to isolate proteins, cells, and DNA.
- Use streptavidin over avidin as it is non-glycosylated and exhibits low levels of nonspecific binding. Avidin is a highly cationic glycoprotein and can cause nonspecific background signal in some applications due to its positively charged residues and oligosaccharide components.
For multicolor experiments, it may be necessary to simultaneously use primary antibodies from the same species. This could cause cross-reactivity between secondary antibodies. This cross-reactivity can be limited by using a biotinylated form of one of the primary antibodies. The biotinylated antibody is then incubated with streptavidin-conjugated fluorophore. This approach will ensure that the streptavidin-conjugate will only bind to biotin, thus limiting cross reactivity.