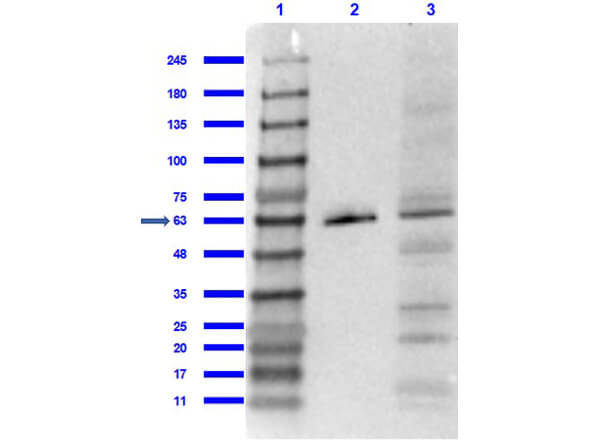
Reactive species like oxidants emerge as byproducts of routine cellular metabolism. They can react with cellular biomolecules and cause damage through oxidative stress. Conditions like Alzheimer's disease (AD) and Parkinson's disease (PD) have been correlated with oxidative stress, underlining its role in certain neurodegenerative disorders (Reviewed by Nandi et al., 2019). Fortunately, cells have evolved an array of protective strategies to counteract reactive oxygen species (ROS), such as hydrogen peroxide. One of these defense mechanisms involves the action of an enzyme called catalase, which breaks down hydrogen peroxide into water and oxygen.
H2O2
Hydrogen peroxide
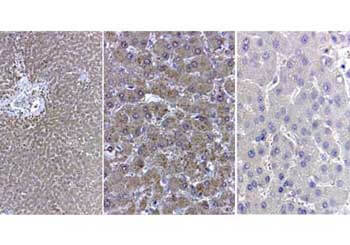
Catalase
→2 H2O
Water
+
O2
Oxygen
Catalase in Alzheimer’s Disease
Alzheimer's disease is characterized by the accumulation of amyloid-β peptides and tau protein in the brain, forming plaques and neurofibrillary tangles, respectively. Research has demonstrated the neurotoxic effects of these amyloid-β peptides in cultured neurons.1 These peptides, derived from the amyloid precursor protein (APP), are initially soluble components present in plasma and cerebrospinal fluid. However, in AD, soluble amyloid-β transforms into insoluble fibrils within plaques through protein-protein interactions.
Notably, in vitro studies have shown that while newly formed amyloid-β is non-toxic, aged forms become harmful to neurons. Evidence suggests that amyloid-β peptides trigger the buildup of hydrogen peroxide in neuroblastoma and hippocampal neuron cultures. This phenomenon is likely attributed to direct binding of amyloid-β to catalase, leading to diminished enzyme activity. These findings have led to a hypothesis proposing that the interaction between catalase and amyloid-β significantly contributes to increased hydrogen peroxide levels within cells, thus linking amyloid-β accumulation and the onset of oxidative stress in Alzheimer's disease.2
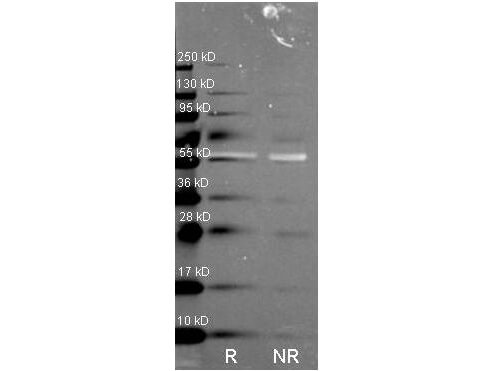
The current prevailing hypothesis regarding the mechanism of amyloid-β-induced oxidative damage suggests that amyloid-β directly binds to catalase, deactivating its catalytic function and subsequently fostering conditions of oxidative stress. Additionally, complete amyloid-β peptides interact with Cu2+ ions at their N-terminal segments, reducing them to Cu+. This amyloid-β Cu+ complex has been shown to promote the generation of hydrogen peroxide. Consequently, catalase exhibits both direct and indirect associations with the pathogenesis of Alzheimer's disease, playing a pivotal role in the intricate interplay between amyloid-β, oxidative stress, and neuronal dysfunction. Rockland supports catalase research with a set of specific antibodies.
Recommended Catalase Products
Catalase in Parkinson’s Disease
Parkinson's disease, an age-related neurological disorder, initially presents as subtle hand tremors that progress to impair overall body movements, significantly diminishing quality of life. As the disease advances, motor control difficulties intensify, characterized by muscle rigidity and slowed movement initiation. The root cause lies in dopamine depletion due to damage to dopamine-producing neurons in the substantia nigra pars compacta (SNpc), resulting in the loss of around 100-200 neurons daily.
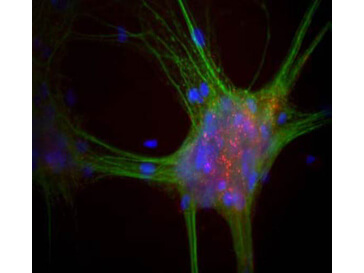
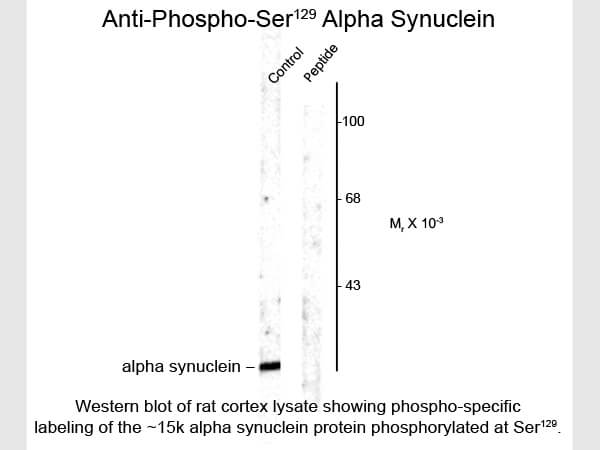
A central player in the disease's cytopathology is the protein α-synuclein. Mutations in the α-synuclein gene lead to the production of a mutant protein that disrupts dopamine synthesis and storage, causing its accumulation within neuron cytoplasm. This aberrant process triggers the auto-oxidation of dopamine, which generates reactive oxygen species like hydrogen peroxide with toxic dopamine-quinone species, culminating in oxidative stress.
Furthermore, mutant α-synuclein also interferes with catalase. This inhibition is attributed to α-synuclein's disruption of peroxisome proliferator-activated receptor γ (PPARγ) transcription activity, which regulates catalase gene expression.3 Collectively, these findings suggest that the altered catalase activity and elevated hydrogen peroxide levels in Parkinson's disease may be attributed, in part, to α-synuclein's indirect impact on catalase expression. This intricate interplay unveils new insights into Parkinson's disease progression and offers potential avenues for therapeutic exploration.
Related Products
References
- Nandi, A., Yan, L. J., Jana, C. K., & Das, N. (2019). Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative medicine and cellular longevity, 2019, 9613090.
- Habib, L. K., Lee, M. T., & Yang, J. (2010). Inhibitors of catalase-amyloid interactions protect cells from beta-amyloid-induced oxidative stress and toxicity. The Journal of biological chemistry, 285(50), 38933–38943.
- Yakunin, E., Kisos, H., Kulik, W., Grigoletto, J., Wanders, R. J., & Sharon, R. (2014). The regulation of catalase activity by PPAR γ is affected by α-synuclein. Annals of clinical and translational neurology, 1(3), 145–159.