Tips for Diluting Antibodies
-
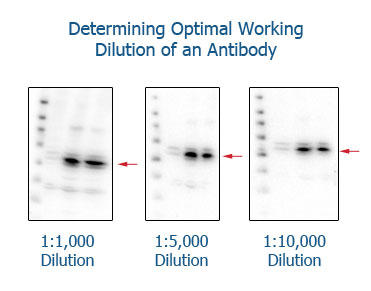
Determining the optimal working concentration of each individual antibody
Correct dilutions of antibodies are best determined by first selecting a fixed incubation time and then making series of dilutions in a titration experiment. For example, if a product datasheet suggests using a 1:1000 dilution for Western blotting, it is recommended to make dilutions of 1:500, 1:1000, 1:2000, 1:4000, and 1:8000. This should determine the optimal dilution for your individual sample conditions.
Similarly for IHC, if the data sheet recommends using a 1:200 dilution, it is suggested to make dilutions of 1:50, 1:100, 1:200, 1:400, and 1:500.
Each dilution should be performed on the same type of sample in order to retain the same experimental conditions.
-
Effect of intrinsic affinity of an antibody on optimal antibody dilution
The rate of binding between antibody and antigen is also dependent on the intrinsic affinity of an antibody. When the titer is held constant, a high-affinity antibody will react faster with the antigen and will provide more intense signal or staining within the same incubation period than an antibody of low affinity. Thus the titers may vary between polyclonal antisera, monoclonal antibodies in culture supernatants, and monoclonal antibodies in ascites fluid.
In practical terms, for polyclonal antisera, the titers may vary from 1:100-1:2000; for chromatographically purified antibodies, the titers may vary from 1:500–1:10,000; for monoclonal antibodies in cell culture supernatants, the titers may vary from 1:10-1:1,000, and for monoclonal antibodies in ascites fluid, the titers may vary from 1:1000-1:100,000.
-
Determining the batch-to-batch consistency
Many antibodies will have comparable batch-to-batch consistency, therefore, in most cases only one titration experiment is required. However, for some antibodies, especially for polyclonal antibodies, when there is a change in the results of the staining between batches of the same antibody, another titration experiment should be performed.
-
Preparing antibody dilutions from concentrated stock solutions
Dilutions are typically expressed as the ratio of the more concentrated stock solution to the total volume of the desired dilution. For example, a 1:10 dilution is made by mixing one part of stock solution with nine parts of diluent. Two fold serial dilutions are prepared by consecutive 1:2 dilutions of the preceding dilution. In order to prepare a small volume of a highly diluted antibody solution, it may be necessary to make it in two steps. For example, to prepare 1.0 ml of a 1:1000 dilution, first prepare 1:10 dilution in100 µL volume (10 µL + 90 µL), and then prepare a 1:100 dilution in 1.0 mL volume by using 10 µL of an intermediate 1:10 dilution (10 µL + 990 µL).
-
Proper pipette usage
The use of adjustable pipettes for preparing dilutions allows for greater flexibility and more precise delivery. Tips supplied or approved by the manufacturer should always be used, since other tips may not fit on the pipette properly. An improper tip seal will cause inaccuracies in the amount of liquid transferred. When aspirating the reagent, the pipette must be held vertically, otherwise too much liquid will be drawn in. When dispensing the sample, the tip should be held at an angle against the container to draw out the liquid. To measure volumes in excess of 1.0 mL, serological or volumetric pipettes should be used.